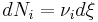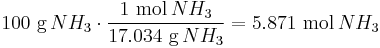Stoichiometry
Stoichiometry (stoi·chi·om·e·try - stoi'kē-ŏm'ĭ-trē) is a branch of chemistry that deals with the quantitative relationships that exist among the reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers. For example, in a reaction that forms ammonia (NH3), exactly one molecule of nitrogen (N2) reacts with three molecules of hydrogen (H2) to produce two molecules of NH3:
- N2 + 3H2 → 2NH3
Reaction stoichiometry describes the quantitative relationships among substances as they participate in chemical reactions. In the example above, reaction stoichiometry describes the 1:3:2 ratio of molecules of nitrogen, hydrogen, and ammonia.
Composition stoichiometry describes the quantitative (mass) relationships among elements in compounds. For example, composition stoichiometry describes the nitrogen to hydrogen (mass) relationship in the compound ammonia.
Stoichiometry can be used to calculate quantities such as the amount of products that can be produced with given reactants and percent yield (the percentage of the given reactant that is made into the product).
A stoichiometric amount or stoichiometric ratio of a reagent is the amount or ratio where, assuming that the reaction proceeds to completion:
- all reagent is consumed,
- there is no shortfall of reagent, and
- no residues remain.
Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume, and can be assumed to be ideal gasses. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio.
While almost all reactions have integer-ratio stoichiometry in amount of matter units (moles, number of particles), some nonstoichiometric compounds are known.
Stoichiometry is based on the law of conservation of mass. The mass of the reactants equals the mass of the products.
Contents |
Etymology
"Stoichiometry" is derived from the Greek words στοιχεῖον (stoicheion, meaning element]) and μέτρον (metron, meaning measure.) In patristic Greek, the word Stoichiometria was used by Nicephorus to refer to the number of line counts of the canonical New Testament and some of the Apocrypha.
Different stoichiometries in competing reactions
Often, more than one reaction is possible given the same starting materials. The reactions may differ in their stoichiometry. For example, the methylation of benzene ( ), through a Friedel-Crafts reaction using
), through a Friedel-Crafts reaction using  as catalyst, may produce singly-methylated
as catalyst, may produce singly-methylated  , doubly-methylated
, doubly-methylated 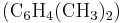 , or still more highly-methylated
, or still more highly-methylated  products, as shown in the following example,
products, as shown in the following example,
In this example, which reaction takes place is controlled in part by the relative concentrations of the reactants.
Stoichiometric coefficient
In layman's terms, the stoichiometric coefficient of any given component is the number of molecules which participate in the reaction as written.
For example, in the reaction CH4 + 2 O2 → CO2 + 2 H2O, the stoichiometric coefficient of CH4 would be 1 and the stoichiometric coefficient of O2 would be 2.
In more technically-precise terms, the stoichiometric coefficient in a chemical reaction system of the i–th component is defined as
or
where Ni is the number of molecules of i, and ξ is the progress variable or extent of reaction (Prigogine & Defay, p. 18; Prigogine, pp. 4–7; Guggenheim, p. 37 & 62).
The extent of reaction ξ can be regarded as a real (or hypothetical) product, one molecule of which is produced each time the reaction event occurs. It is the extensive quantity describing the progress of a chemical reaction equal to the number of chemical transformations, as indicated by the reaction equation on a molecular scale, divided by the Avogadro constant (it is essentially the amount of chemical transformations). The change in the extent of reaction is given by dξ = dnB/nB, where nB is the stoichiometric number of any reaction entity B (reactant or product) an dnB is the corresponding amount.[1]
The stoichiometric coefficient νi represents the degree to which a chemical species participates in a reaction. The convention is to assign negative coefficients to reactants (which are consumed) and positive ones to products. However, any reaction may be viewed as "going" in the reverse direction, and all the coefficients then change sign (as does the free energy). Whether a reaction actually will go in the arbitrarily-selected forward direction or not depends on the amounts of the substances present at any given time, which determines the kinetics and thermodynamics, i.e., whether equilibrium lies to the right or the left.
If one contemplates actual reaction mechanisms, stoichiometric coefficients will always be integers, since elementary reactions always involve whole molecules. If one uses a composite representation of an "overall" reaction, some may be rational fractions. There are often chemical species present that do not participate in a reaction; their stoichiometric coefficients are therefore zero. Any chemical species that is regenerated, such as a catalyst, also has a stoichiometric coefficient of zero.
The simplest possible case is an isomerism
in which νB = 1 since one molecule of B is produced each time the reaction occurs, while νA = −1 since one molecule of A is necessarily consumed. In any chemical reaction, not only is the total mass conserved, but also the numbers of atoms of each kind are conserved, and this imposes a corresponding number of constraints on possible values for the stoichiometric coefficients. Of course, only a small subset of the possible atomic rearrangements will occur.
There are usually multiple reactions proceeding simultaneously in any natural reaction system, including those in biology. Since any chemical component can participate in several reactions simultaneously, the stoichiometric coefficient of the i–th component in the k–th reaction is defined as
so that the total (differential) change in the amount of the i–th component is
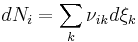 .
.
Extents of reaction provide the clearest and most explicit way of representing compositional change, although they are not yet widely used.
With complex reaction systems, it is often useful to consider both the representation of a reaction system in terms of the amounts of the chemicals present { Ni } (state variables), and the representation in terms of the actual compositional degrees of freedom, as expressed by the extents of reaction { ξk }. The transformation from a vector expressing the extents to a vector expressing the amounts uses a rectangular matrix whose elements are the stoichiometric coefficients [ νi k ].
The maximum and minimum for any ξk occur whenever the first of the reactants is depleted for the forward reaction; or the first of the "products" is depleted if the reaction as viewed as being pushed in the reverse direction. This is a purely kinematic restriction on the reaction simplex, a hyperplane in composition space, or N‑space, whose dimensionality equals the number of linearly-independent chemical reactions. This is necessarily less than the number of chemical components, since each reaction manifests a relation between at least two chemicals. The accessible region of the hyperplane depends on the amounts of each chemical species actually present, a contingent fact. Different such amounts can even generate different hyperplanes, all of which share the same algebraic stoichiometry.
In accord with the principles of chemical kinetics and thermodynamic equilibrium, every chemical reaction is reversible, at least to some degree, so that each equilibrium point must be an interior point of the simplex. As a consequence, extrema for the ξ's will not occur unless an experimental system is prepared with zero initial amounts of some products.
The number of physically-independent reactions can be even greater than the number of chemical components, and depends on the various reaction mechanisms. For example, there may be two (or more) reaction paths for the isomerism above. The reaction may occur by itself, but faster and with different intermediates, in the presence of a catalyst.
The (dimensionless) "units" may be taken to be molecules or moles. Moles are most commonly used, but it is more suggestive to picture incremental chemical reactions in terms of molecules. The N's and ξ's are reduced to molar units by dividing by Avogadro's number. While dimensional mass units may be used, the comments about integers are then no longer applicable.
Stoichiometry matrix
In complex reactions, stoichiometries are often represented in a more compact form called the stoichiometry matrix. The stoichiometry matrix is denoted by the symbol,  .
.
If a reaction network has  reactions and
reactions and  participating molecular species then the stoichiometry matrix will have corresponding
participating molecular species then the stoichiometry matrix will have corresponding  columns and
columns and  rows.
rows.
For example, consider the system of reactions shown below:
- S1 → S2
- 5S3 + S2 → 4S3 + 2S2
- S3 → S4
- S4 → S5.
This systems comprises four reactions and five different molecular species. The stoichiometry matrix for this system can be written as:
where the rows correspond to S1, S2, S3, S4 and S5, respectively. Note that the process of converting a reaction scheme into a stoichiometry matrix can be a lossy transformation, for example, the stoichiometries in the second reaction simplify when included in the matrix. This means that it is not always possible to recover the original reaction scheme from a stoichiometry matrix.
Often the stoichiometry matrix is combined with the rate vector, v to form a compact equation describing the rates of change of the molecular species:
Gas stoichiometry
Gas stoichiometry is the quantitative relationship (ratio) between reactants and products in a chemical reaction with reactions that produce gases. Gas stoichiometry applies when the gases produced are assumed to be ideal, and the temperature, pressure, and volume of the gases are all known. The ideal gas law is used for these calculations. Often, but not always, the standard temperature and pressure (STP) are taken as 0°C and 1 bar and used as the conditions for gas stoichiometric calculations.
Gas stoichiometry calculations solve for the unknown volume or mass of a gaseous product or reactant. For example, if we wanted to calculate the volume of gaseous NO2 produced from the combustion of 100 g of NH3, by the reaction:
- 4NH3 (g) + 7O2 (g) → 4NO2 (g) + 6H2O (l)
we would carry out the following calculations:
There is a 1:1 molar ratio of NH3 to NO2 in the above balanced combustion reaction, so 5.871 mol of NO2 will be formed. We will employ the ideal gas law to solve for the volume at 0 °C (273.15 K) and 1 atmosphere using the gas law constant of R = 0.08206 L · atm · K−1 · mol−1 :
Gas stoichiometry often involves having to know the molar mass of a gas, given the density of that gas. The ideal gas law can be re-arranged to obtain a relation between the density and the molar mass of an ideal gas:
 and
and 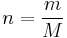
and thus:
| where: | |
 |
= absolute gas pressure |
|---|---|
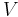 |
= gas volume |
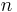 |
= number of moles |
 |
= universal ideal gas law constant |
 |
= absolute gas temperature |
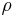 |
= gas density at  and and  |
 |
= mass of gas |
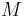 |
= molar mass of gas |
Stoichiometric air-fuel ratios of common fuels
| Fuel | By mass | By volume [2] | Percent fuel by mass |
|---|---|---|---|
| Gasoline | 14.7 : 1 | — | 6.8% |
| Natural gas | 17.2 : 1 | 9.7 : 1 | 5.8% |
| Propane (LP) | 15.5 : 1 | 23.9 : 1 | 6.45% |
| Ethanol | 9 : 1 | — | 11.1% |
| Methanol | 6.4 : 1 | — | 15.6% |
| Hydrogen | 34 : 1 | 2.39 : 1 | 2.9% |
| Diesel | 14.6 : 1 | 0.094 : 1 | 6.8% |
Gasoline engines are run at stoichiometric air-to-fuel ratio, because gasoline is so volatile that mixes properly with the air. Diesel engines, in contrast, run lean, with more air available than simple stoichiometry would require. Diesel fuel is heavier and does not burn immediately to give gaseous products. Thus, it would form soot (black smoke) at stoichiometric ratio.
References
- ↑ IUPAC Compendium of Chemical Terminology 2nd Edition (1997)
- ↑ North American Mfg. Co.: "North American Combustion Handbook", 1952
- Ilya Prigogine & R. Defay, translated by D.H. Everett; Chapter IV (1954). Chemical Thermodynamics. Longmans, Green & Co. Exceptionally clear on the logical foundations as applied to chemistry; includes non-equilibrium thermodynamics.
- Ilya Prigogine (1967). Thermodynamics of Irreversible Processes, 3rd ed.. Interscience: John Wiley & Sons. A simple, concise monograph. Library of Congress Catalog No. 67-29540
- E.A. Guggenheim (1967). Thermodynamics: An Advanced Treatment for Chemists and Physicists, 5th ed.. North Holland; John Wiley & Sons (Interscience). A remarkably astute treatise. Library of Congress Catalog No. 67-20003
- Zumdahl, Steven S. Chemical Principles. Houghton Mifflin, New York, 2005, pp 148–150.
External links
- Engine Combustion primer from the University of Plymouth
- Free Stoichiometry Tutorials from Carnegie Mellon's ChemCollective